ST PAUL, Minn. — Wednesday, April 14
- MDH reports 681 Minnesotans hospitalized with COVID as of Tuesday
- FDA, CDC recommend "pause" on Johnson & Johnson vaccine
- 2 community testing sites closing early due to curfew
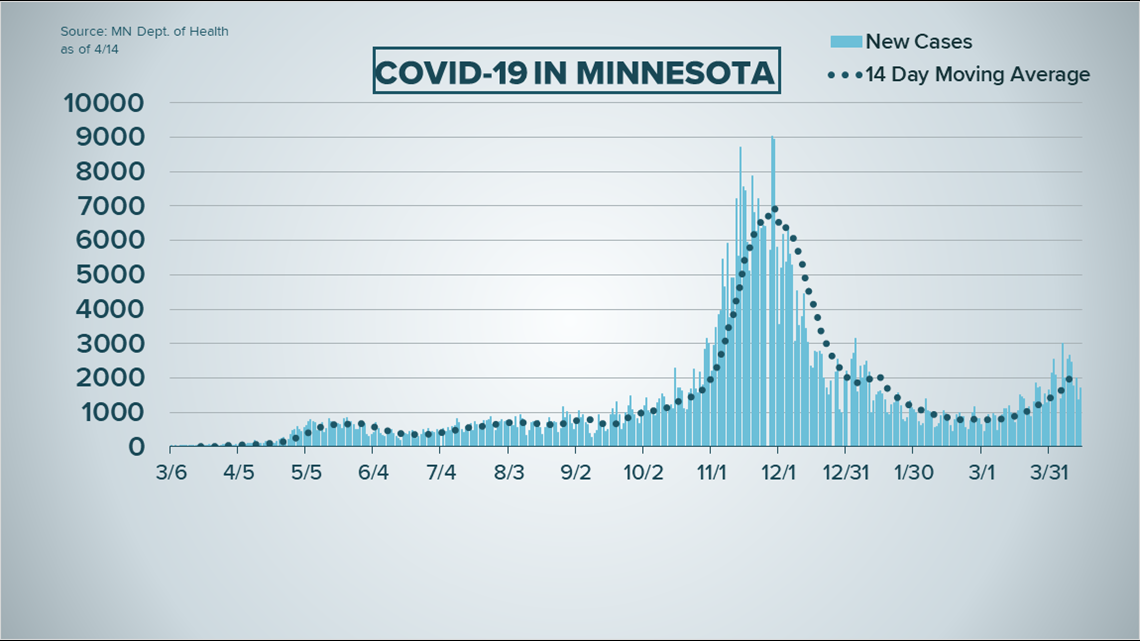
- Another day of new COVID cases near 2,000
- 2 Minnesota colleges will not require vaccinations for students to return to campus, others deciding
COVID-19 cases took a slight dip, according to data released Wednesday by the Minnesota Department of Health, while hospitalizations remain at a concerning level.
Health officials say 1,715 new infections were reported in the last day, bringing the state total to 547,101 since the pandemic began. The new cases are based on results from 23,669 tests (19,821 PCR, 3,848 antigen) processed in private and state labs.

Hospitalization rates remain high, with 681 people being treated on an in-patient basis across Minnesota as of Wednesday. MDH says 164 of those patients are in ICU. Bed availability in the Twin Cities is low, with just 61 non-ICU beds (1.7% vacancy) currently open.
In total, 28,636 people have been hospitalized since the virus arrived in the state. State health officials say 522,843 people who at one time tested positive for COVID have improved to the point they no longer need to be isolated.
An additional 16 people have died of COVID, bringing fatalities to 6,978. Of those deaths 4,320 (just under 62%) are tied to assisted living or long-term care facilities.
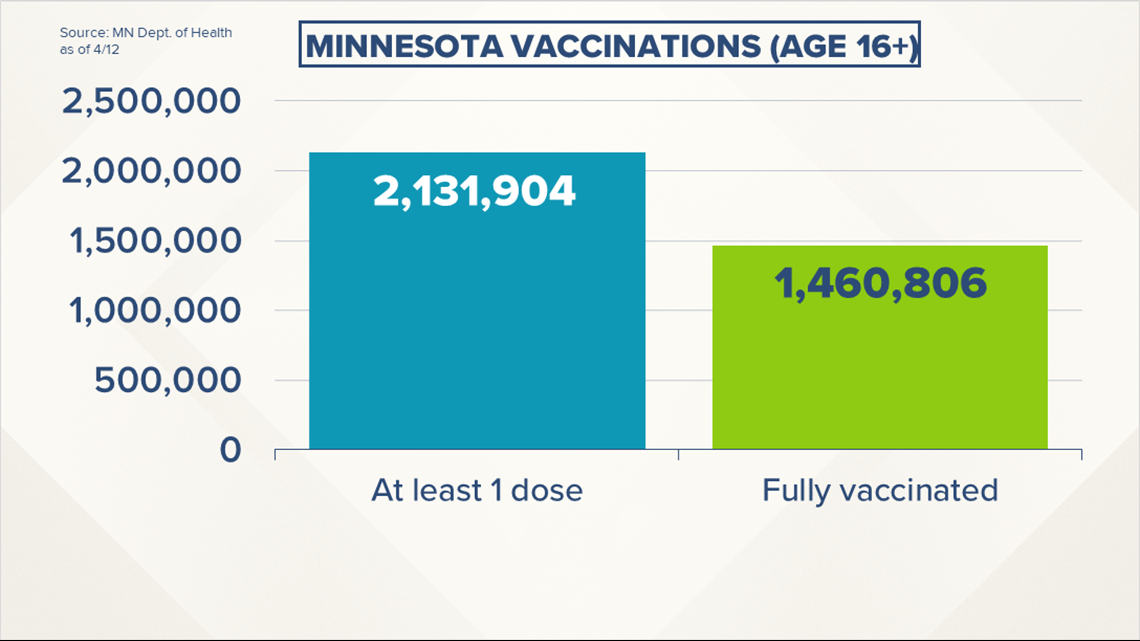
The number of Minnesotans considered fully vaccinated is getting closer and closer to 1.5 million. As of Tuesday 1,460,806 people had completed the COVID series (whether one shot or two), 33.1% of Minnesota's eligible population of people age 16 and older. The state's vaccine dashboard says 2,131,826 residents have been immunized with at least one shot.

Young adults make up the largest group of the state's COVID cases, with those 20 to 24 accounting for 53,468 cases and four fatalities. The virus has been most deadly for people between 85 and 89, with 1,302 fatalities in 6,555 diagnosed cases.
The state's top four counties in terms of population have also recorded the most COVID activity: Hennepin County reports 113,983 cases and 1,672 deaths, followed by Ramsey County with 47,188 cases and 850 deaths, Dakota County with 41,764 cases and 418 deaths, and Anoka County with 37,816 cases and 415 fatalities.
Tuesday, April 13
11 a.m.
Hospitalizations related to COVID-19 are at a level not seen in the state since mid-January, according to data released Tuesday by the Minnesota Department of Health (MDH).
Reports from hospitals across the state say 676 people are being treated for symptoms of the virus on an in-patient basis, the highest single-day number since Jan. 11 when 692 were hospitalized with COVID. MDH says 159 of the hospitalizations reported Tuesday are being treated in the ICU.
MDH officials have stated in calls with reporters in recent days they believe the increase in hospitalizations is tied to the spread of the B.1.1.7 variant, which spreads more easily and triggers more severe COVID symptoms.
Total hospitalizations have now risen to 28,509, with 5,830 of those patients requiring ICU care. Health officials say 520,800 people who once tested positive for the virus are healthy enough to no longer require isolation.
The state vaccine dashboard says as of Sunday 2,102,859 Minnesotans have received at least one dose of vaccine, 47.7% of those eligible to be immunized (age 16 and older). MDH says 1,435,224 people have completed their vaccine series, whether one or two shots, and are considered fully vaccinated.
10 a.m.
State officials are advising health providers to follow the federally recommended "pause" on use of the Johnson & Johnson COVID-19 vaccine.
Both the U.S. Centers for Disease Control and Prevention (CDC) and Food and Drug Administration urged Tuesday that no J & J vaccines be administered while investigators review data concerning an "extremely rare" blood clot reported by six people who received that vaccine in the U.S.
Minnesota Commissioner of Health Jan Malcolm said Tuesday that until that process is complete, state health officials have advised providers to stop using the Johnson & Johnson vaccine out of "an abundance of caution."
“While this issue appears to be extremely rare, CDC and FDA are acting in a very cautious manner that underscores our commitment to vaccine safety,” Commissioner Malcolm said. “We will be closely monitoring the federal review process and use that information to help guide our efforts here in Minnesota in the days ahead.”
The state says the Johnson & Johnson vaccine represents only 6.6% percent of the total doses Minnesota has received to date, so the pause is not expected to dramatically slow the pace of vaccinations in the state.
While Minnesota officials say they are not aware of any serious side effects occurring among the more than 184,000 Minnesotans who have received this vaccine, anyone who was immunized with the Johnson & Johnson vaccine who develops severe headache, abdominal pain, leg pain, or shortness of breath within three weeks after vaccination should contact their health care provider immediately.
Anyone who currently has an appointment to receive the Johnson & Johnson vaccine is advised to watch for a notification from their provider about canceling, postponing, or rescheduling the appointment.
Vaccination appointments can be found by signing up for the Vaccine Connector, by using the state Vaccine Locator map, or checking with local pharmacies or your health care provider.
7 a.m.
Federal officials are recommending Johnson & Johnson's one-dose COVID-19 vaccine be put on "pause" while the cases of at least six women who developed potentially dangerous blood clots are investigated.
The Centers for Disease Control and Prevention and the Food and Drug Administration confirmed the investigation in a joint statement, adding that reduced platelet counts have also been reported after receiving the Johnson & Johnson product.
More than 6.8 million doses of the J&J vaccine have been administered in the U.S., but the state of Minnesota vaccine dashboard indicates it has played a fairly minor role here. Of well over 3 million doses that have been administered as of Sunday, just 166,326 of them have been Johnson & Johnson, just 5% of the state total.
U.S. federal distribution channels, including mass vaccination sites, will pause the use of the J&J shot and states and other providers are expected to follow. A White House spokesman downplayed the impact of the development on the nationwide vaccination effort.
"This announcement will not have a significant impact on our vaccination plan: Johnson & Johnson vaccine makes up less than 5 percent of the recorded shots in arms in the United States to date," said White House COVID-19 Response Coordinator Jeff Zients in a released statement. "Based on actions taken by the President earlier this year, the United States has secured enough Pfizer and Moderna doses for 300 million Americans. each week, and in fact this week we will make available 28 million doses of these vaccines. This is more than enough supply to continue the current pace of vaccinations of 3 million shots per day, and meet the President’s goal of 200 million shots by his 100th day in office - and continue on to reach every adult who wants to be vaccinated."
No announcement on Minnesota's course of action following the vaccine announcement has yet been made.

