GOLDEN VALLEY, Minn. — We heard A LOT about heat index and dew point last week, but how exactly do those things work to make us feel uncomfortable, or worse yet... cause us to overheat?
The dew point is the direct measure of how much water is in the air. It's a temperature that the air must cool to in order for water vapor to condense into visible form (i.e. dew) but we can convert it to X number of pounds of water per X number of pounds of air (or grams in scientific terms).
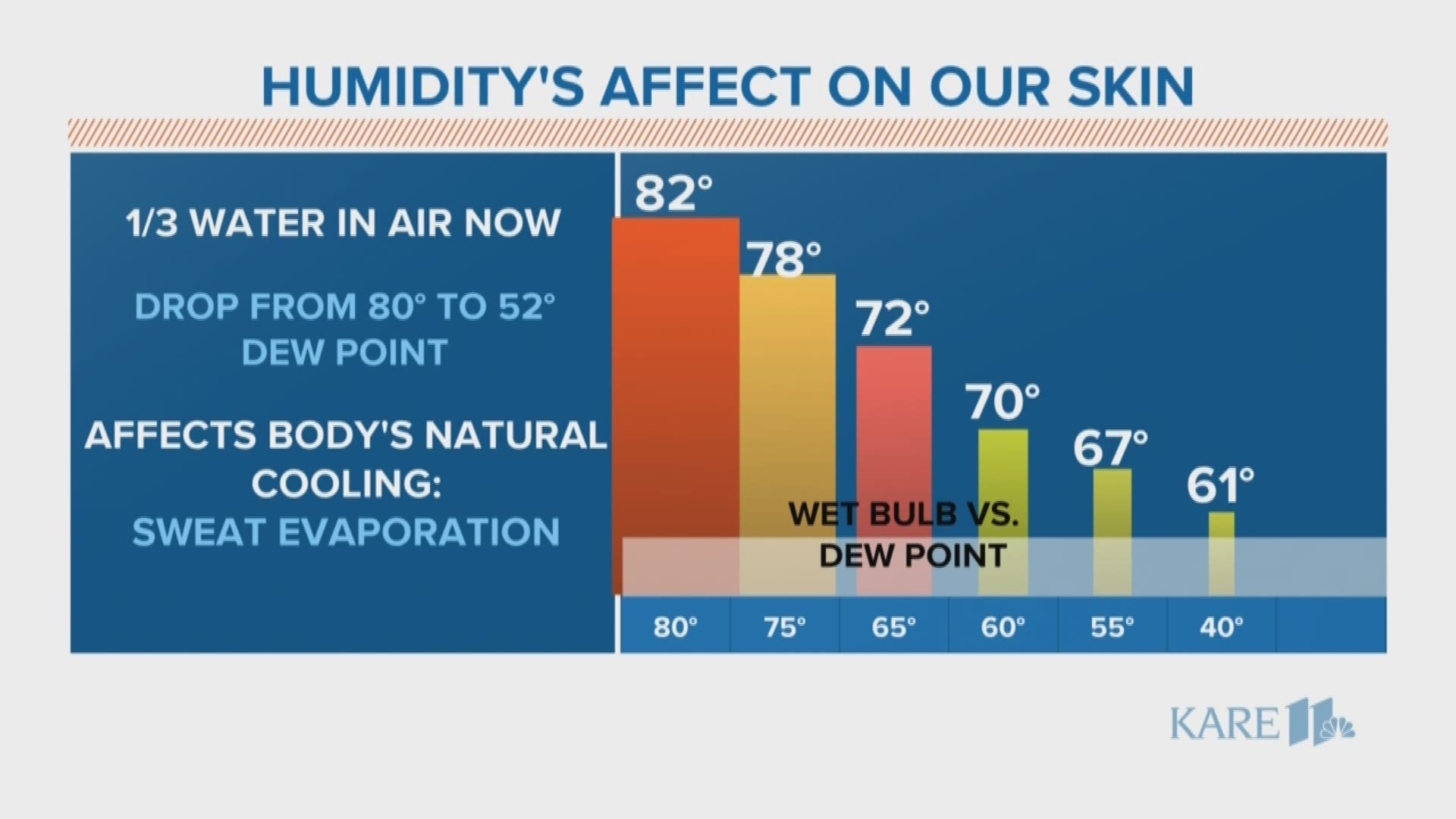
The dew point topped out at 80 last Friday afternoon which equates to about 5.5 lbs of water per 100 lbs of air, while early Monday the dew point was around 50 which equates to just 1.8 lbs of water per 100 lbs of air: That means there's 1/3 of the water content in the air today as compared to last Friday!
I'm going to throw another temperature at you: The wet bulb temperature. It's a measurement of the cooling effect of evaporation. We literally take a piece of wet gauze place it on the bulb of a thermometer and 'sling' it around to get the water to evaporate. The temperature of the bulb will then cool.
Evaporation is a cooling process because it takes a lot of energy to evaporate water molecules, and that lowers the surrounding temperature. Our bodies take advantage of this to create a natural cooling process: We sweat, and that sweat evaporates, cooling our skin's surface.
This cooling process becomes less and less efficient as the air becomes more humid. Let's take an example of 90 degree air (air temp). With Friday's 80 degree dew point the wet bulb temp, the temperature at which could be achieved through evaporation, is only 82. Hence, we'd overheat. But with a 40 degree dew point (dry air- think Palm Springs or Phoenix) the wet bulb temp is 61- natural AC!